How Does It Work?
The L360 Thigh System is intended to assist extension or flexion in adult individuals with muscle weakness related to disease or injury. By electrically stimulating muscles in the affected leg to extension or flexion, the system also may improve the individual’s gait.
3D Motion Detection
3D motion detection deploys stimulation to match your daily activities and lifestyle.


Unique User Modes
Targeted stimulation in three modes―Training, Cycle Training, and Gait―provides dynamic recovery.
Comfort Cuff
The flexible, comfortable, durable orthosis moves with you to support your active life.

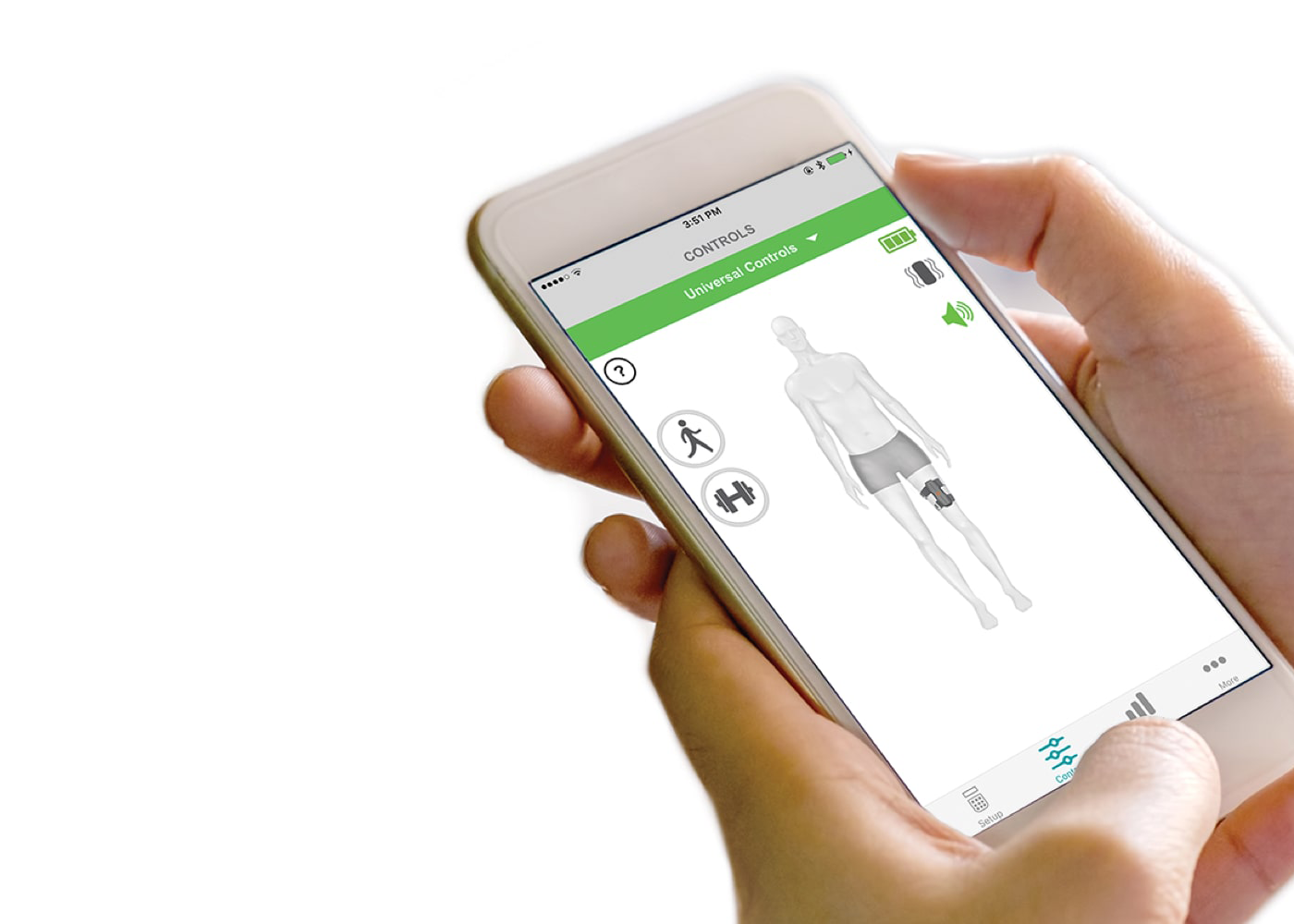
myBioness™ App
The home user app allows you to control the system, set goals, and monitor activity, so you can be engaged with your recovery.
More Questions?
Have questions about the L360 Thigh System? Check out common questions and concerns on the FAQs page.
Contact us to schedule a demo with L360 Thigh System or if you have questions about other Bioness products.
Indications for Use: The L360 Thigh System is intended to assist knee flexion or extension in adult individuals with muscle weakness related to upper motor neuron disease/injury (e.g., stroke, damage to pathways to the spinal cord). The L360 Thigh System electrically stimulates muscles in the affected leg to provide knee flexion or extension; thus, it also may improve the individual’s gait.
The L360 Thigh System may also facilitate muscle re-education, prevent/retard disuse atrophy, maintain or increase joint range of motion, increase local blood flow, provide early post-surgical quadriceps and hamstring strengthening, improve post-surgical knee stability secondary to quadriceps and hamstring strengthening, and relax muscle spasms.
L360 Thigh System is contraindicated in patients with a demand-type cardiac pacemaker, defibrillator or any electrical implant. Do not use the system on a leg where metallic implant is directly underneath the electrodes, a cancerous lesion is present or suspected, or on a leg with regional disorder (e.g., fracture or dislocation) which could be adversely affected by motion from the stimulation. Use caution in patients with diagnosed or suspected cardiac problems or epilepsy.
Full prescribing information can be found in product labeling or at: www.bionessrehab.com/l360/safety-information.

