Resources
Select any H200 Wireless resource below to open it.
H200 Wireless User Guide
- Safety information
- H200 Wireless components
- Setup, usage, maintenance
H200 Wireless User Reference Card
- Quick reference guide
- Using the H200 Wireless
- Control unit displays
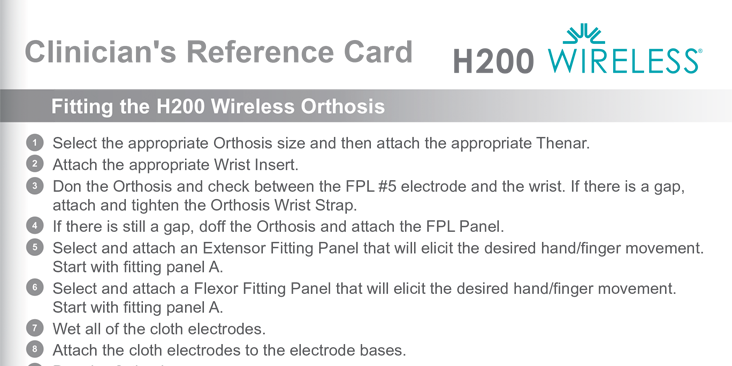
H200 Wireless Clinician Reference Card
- Fitting the orthosis
- Adjusting stimulation and settings
- Using clinical mode
Multiple Sclerosis
- National Multiple Sclerosis Society
- Multiple Sclerosis Foundation
Stroke
- American Stroke Association
- National Institute of Neurological Disorders and Stroke
Spinal Cord Injury
- Christopher & Dana Reeve Foundation
- United Spinal Association
Traumatic Brain Injury
- Brain Injury Association of America
- National Institute of Neurological Disorders and Stroke
Control Unit Registration
Orthosis Registration
Step-by-step video instructions to register your H200 Wireless orthosis.
System Sync Check
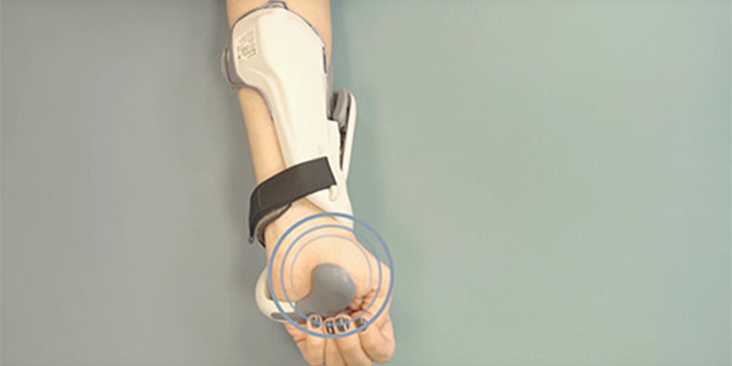
Putting on/taking off the H200 Wireless
Putting on/Taking off the H200 Wireless" video resource: "Video with step-by-step instructions for putting on and taking off the H200 Wireless.
H200 Wireless Clinician Brochure
- Benefits of FES
- Clinical evidence
- H200 Wireless components
More Questions?
800-211-9136 option 3
Our in-house Technical and Clinical Support team is available via phone Monday through Friday, from 8am – 5pm (Pacific Standard Time). Please call us at 800-211-9136 option 3. Or complete our online Contact Us form.
Contact us to schedule a demo with H200 Wireless or if you have questions about other Bioness Medical, Inc. products.
Indications for Use
The H200 Wireless System is an electrical stimulation device indicated for the following uses:
- Functional Electrical Stimulation (FES)
- Improvement of hand function and active range of motion in patients with hemiplegia due to stroke or upper limb paralysis due to C5 spinal cord injury.
- NeuroMuscular Electrical Stimulation (NMES)
- Maintenance and/or increase of hand range of motion; prevention and/or retardation of disuse atrophy; increase in local blood circulation; reduction of muscle spasm; re-education of muscles.
H200 Wireless is contraindicated in patients with a demand-type cardiac pacemaker, defibrillator or any electrical implant. Do not use the system on a hand where metallic implant is directly underneath the electrodes, a cancerous lesion is present or suspected, or on a hand with regional disorder (e.g., fracture or dislocation) which could be adversely affected by motion from the stimulation. Use caution in patients with diagnosed or suspected cardiac problems or epilepsy.
Full prescribing information can be found in product labeling or at: www.bionessrehab.com/h200/safety-information/.
Instructions for Use / Patient Guides and Reference Cards can be made available upon request.
Contact [email protected] or 800-211-9136 to request an electronic copy.