Research Studies
Highlights of the clinical evidence about the H200 Wireless Hand Rehabilitation System:

Use of the H200 Wireless benefits patients 0-3 months post injury
Alon showed that a 12-week task-specific training protocol incorporating FES resulted in better functional recovery of the upper extremity in stroke survivors with little to no hand movement than task-specific training alone.1 (n=15)
- Subjects practiced one hour per day, 5 days/week as inpatients and after discharge continued with one hour per day, 5 days/week at home, unsupervised
- All patients demonstrated improvement in hand function at 12 weeks with significant differences between the control (non-H200 Wireless) and H200 Wireless groups (p=0.049)
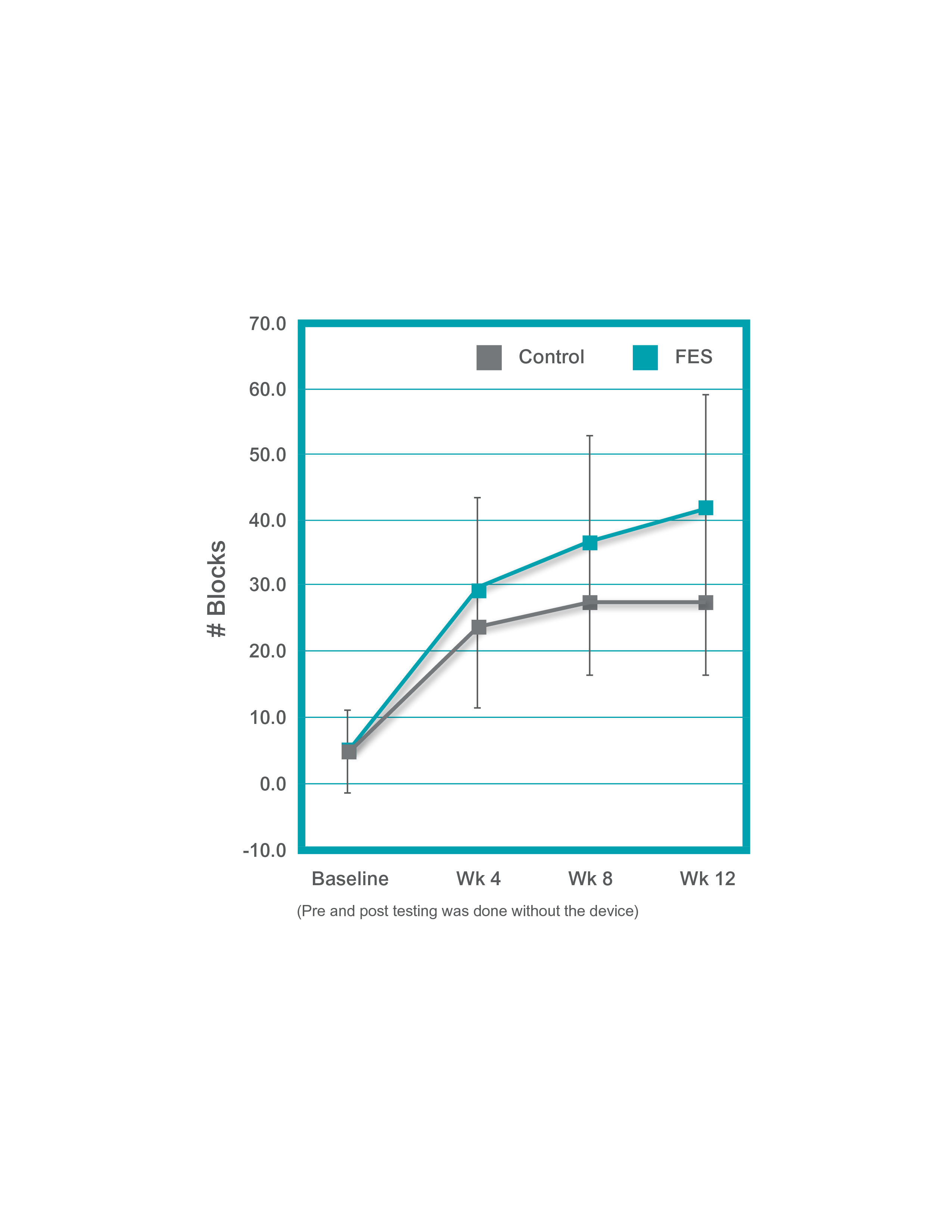
Longer repetitive practice produces significant benefits
Alon showed that a 12-week task-specific training protocol incorporating FES resulted in better functional recovery of the upper extremity in stroke survivors with little to no hand movement than task-specific training alone.1 (n=15)



Patients 3-6 months post injury see compelling benefits with home use
A subacute study by Ring assessed daily use of the H200 Wireless and showed significantly improved outcomes vs. a control group.3 (n=22)
- A six week home based program resulted in significant reduction in spasticity throughout the upper extremity and active range of motion in the shoulder and wrist. (p<0.05)
- In individuals with partial active motion of the hand, greater functional recovery of the upper extremity was demonstrated by statistically significant differences in 4* functional hand tests
Type I: Patients with no active voluntary motion at the fingers and wrist. (n=10)
Type II: Patients with partial active voluntary range of motion. (n=12)
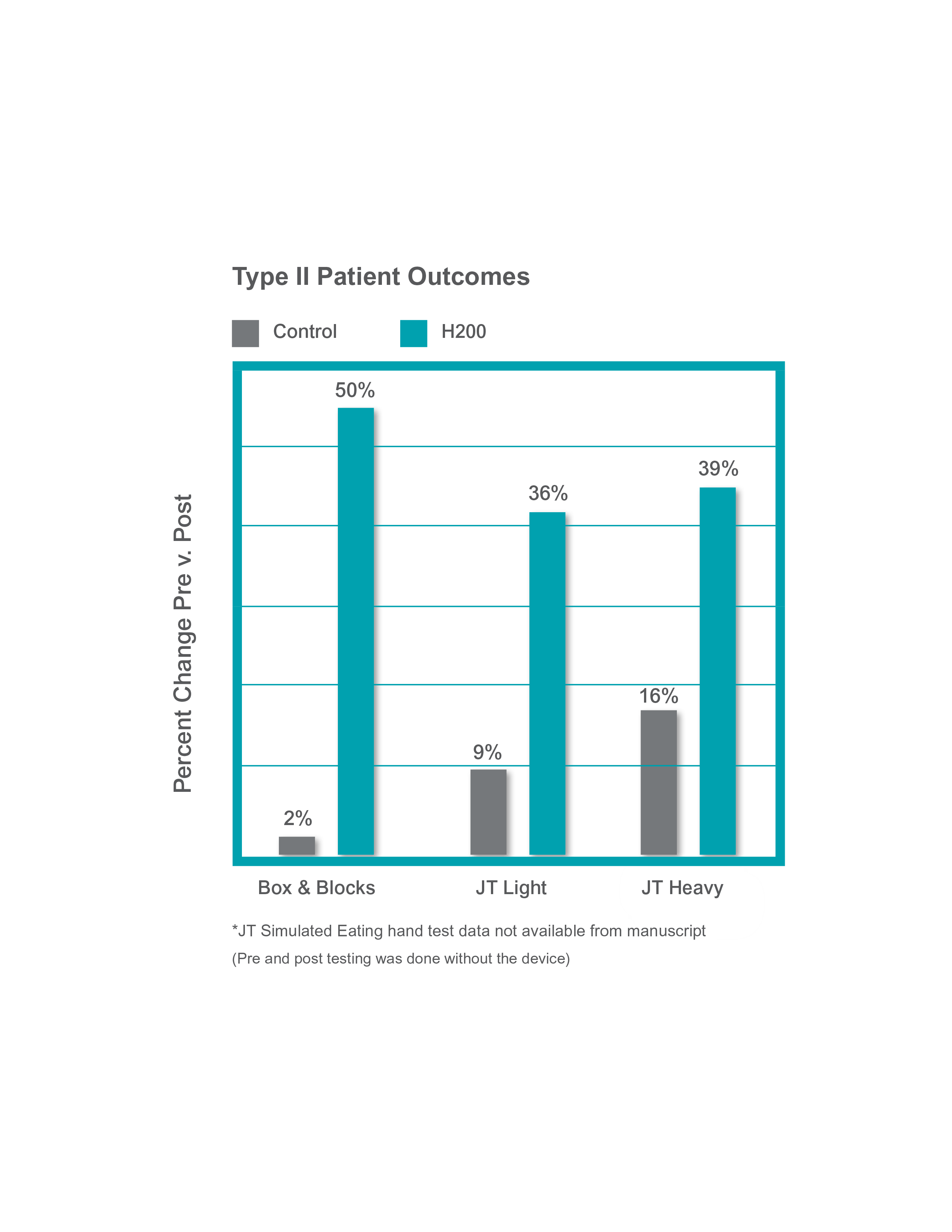
Daily home use improves hand function in the chronic patient population
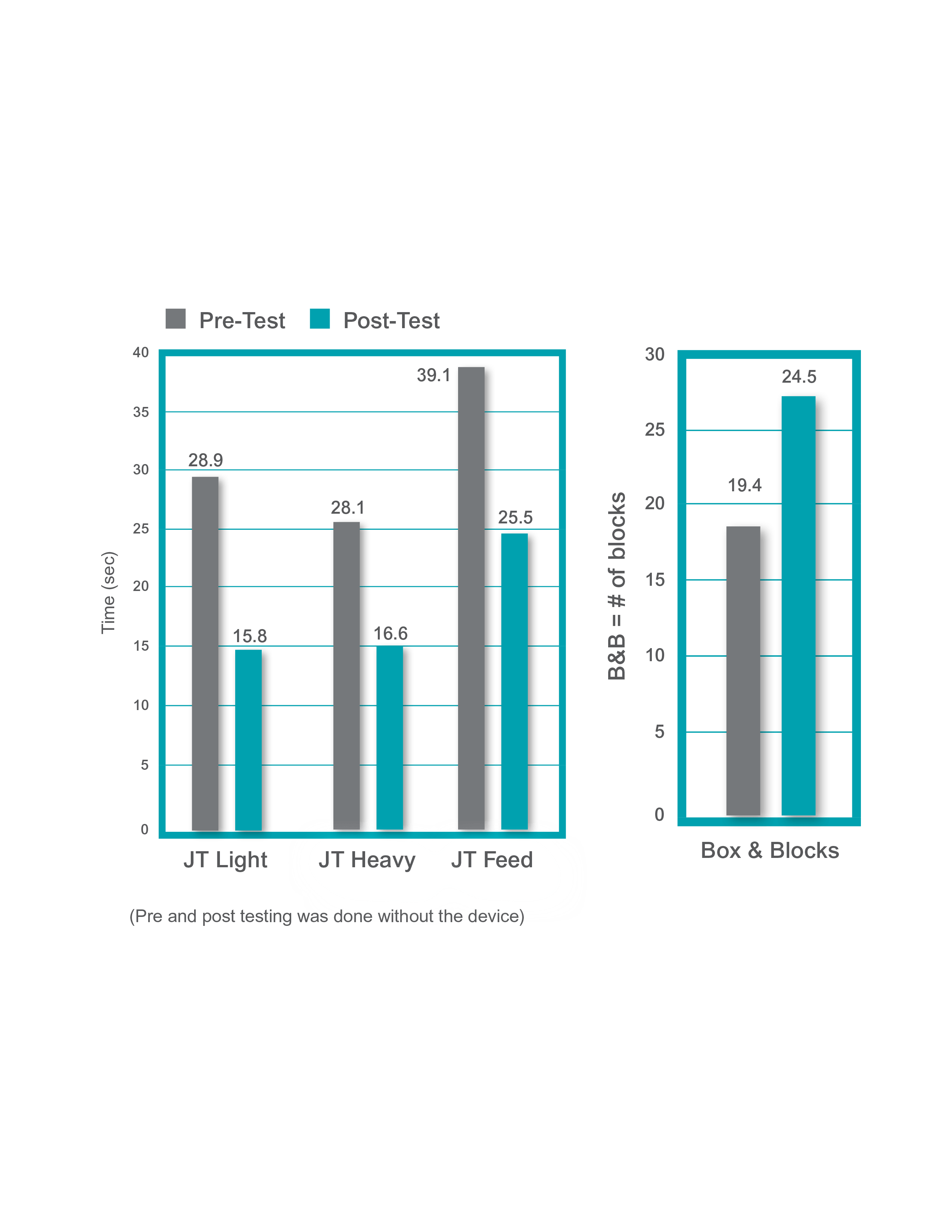
A study by Alon tested the efficacy of a home-based H200 Wireless program in chronic stroke subjects and showed statistically significant improvements in functional outcomes.2 (n=77)
- A five-week home-based training program that combined H200 Wireless use with exercise ~90 minutes/day showed significant improvements in functional outcomes post-test versus baseline (p<0.01)
- Mean time since stroke = 3.3 years

Practice Support and Resources
Check out downloadable materials and support to help you get the most from your H200 Wireless Hand Rehabilitation System.
Contact us to schedule a demo with H200 Wireless or if you have questions about other Bioness products.
- Alon G, McBride K, Ring H. Improving selected hand functions using a noninvasive neuroprosthesis in persons with chronic stroke. J Stroke Cereb Dis. 2002;11(2):99-106. doi:https://doi.org/10.1053/jscd.2002.127107
- Alon G, Sunnerhagen KS, Geurts AC, and Ohry A. A home-based, self-administered stimulation program to improve selected hand functions of chronic Stroke. NeuroRehabilitation. 2003;18(3):215-225. doi:10.3233/NRE-2003-18306
- Ring H, Rosenthal N. Controlled study of neuroprosthetic functional electrical stimulation in sub-acute post-stroke rehabilitation. J Rehabil Med. 2005:37(1):32-6. doi:10.1080/16501970410035387
Indications for Use:
The H200 Wireless System is an electrical stimulation device indicated for the following uses:
- Functional Electrical Stimulation (FES)
- Improvement of hand function and active range of motion in patients with hemiplegia due to stroke or upper limb paralysis due to C5 spinal cord injury.
- NeuroMuscular Electrical Stimulation (NMES)
- Maintenance and/or increase of hand range of motion; Prevention and/or retardation of disuse atrophy; Increase in local blood circulation; Reduction of muscle spasm; Re-education of muscles.
H200 Wireless is contraindicated in patients with a demand-type cardiac pacemaker, defibrillator or any electrical implant. Do not use the system on a hand where metallic implant is directly underneath the electrodes, a cancerous lesion is present or suspected, or on a hand with regional disorder (e.g., fracture or dislocation) which could be adversely affected by motion from the stimulation. Use caution in patients with diagnosed or suspected cardiac problems or epilepsy.
Full prescribing information can be found in product labeling or at www.bionessrehab.com/h200/safety-information/.
Instructions for Use / Patient Guides and Reference Cards can be made available upon request.
Contact [email protected] or 800-211-9136 to request an electronic copy.

