L360 Thigh System Resources
Select any L360 Thigh System resource below to open it.
L360 User’s Guide
- Safety information
- Setup, operation, and maintenance
- Full details about the L360 Thigh System
myBionessTM App: Phone Compatibility List
- Android operating system versions
- Apple iPhone versions
- Compatibility FAQs
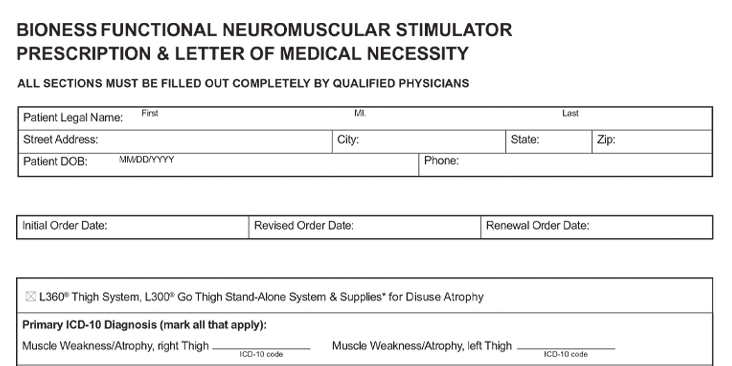
L360 Clinician’s Guide
Product Brochure
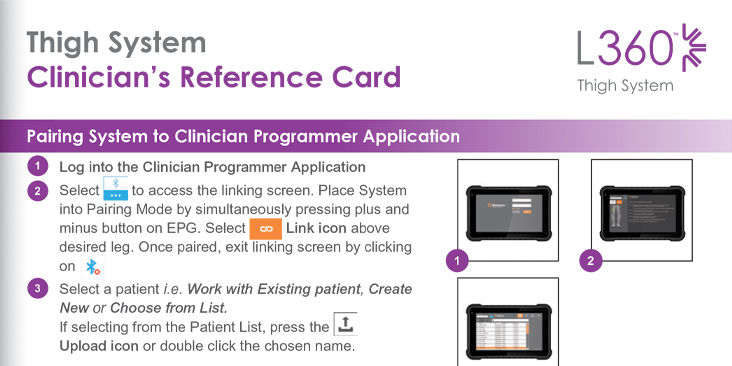
Clinician’s Reference Card
L360 Brochure
L360 Technology Introduction
Surgeon Associations
- American Academy of Orthopedic Surgeons
- American Association of Hip and Knee Surgeons
Osteoarthritis
- Arthritis Foundation
- Osteoarthritis Action Alliance
- National Institute of Arthritis and Musculoskeletal and Skin Diseases
- CDC Podcast: Healthy Living with Arthritis
- Partners of CDC's Arthritis Program
ACL
- American Orthopedic Society for Sports Medicine: Resources with evidence-based diagnosis and treatment for athletes
- JOSPT Insights (Podcast): ACL Graft Types and Rehabilitation
Journals
- Journal of Orthopaedic & Sports Physical Therapy
- American Journal of Sports Medicine
- Orthopaedic Journal of Sports Medicine
- Video Journal of Sports Medicine
Technical Support
Nothing found.
More Questions?
Our in-house Technical and Clinical Support team is available via [email protected]. Or complete our online Contact Us form.
Contact us to schedule a demonstration of the L360 Thigh System or to get more information about Bioness products.
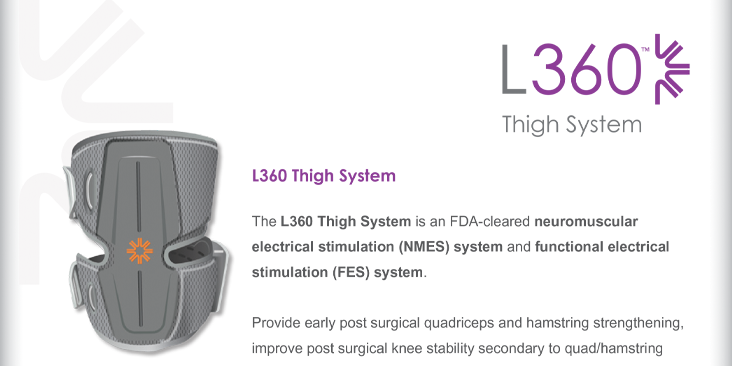
Indications for Use: The L360 Thigh System is intended to assist knee flexion or extension in adult individuals with muscle weakness related to upper motor neuron disease/injury (e.g., stroke, damage to pathways to the spinal cord). The L360 Thigh System electrically stimulates muscles in the affected leg to provide knee flexion or extension; thus, it also may improve the individual’s gait.
The L360 Thigh System may also facilitate muscle re-education, prevent/retard disuse atrophy, maintain or increase joint range of motion, increase local blood flow, provide early post-surgical quadriceps and hamstring strengthening, improve post-surgical knee stability secondary to quadriceps and hamstring strengthening, and relax muscle spasms.
L360 Thigh System is contraindicated in patients with a demand-type cardiac pacemaker, defibrillator or any electrical implant. Do not use the system on a leg where metallic implant is directly underneath the electrodes, a cancerous lesion is present or suspected, or on a leg with regional disorder (e.g., fracture or dislocation) which could be adversely affected by motion from the stimulation. Use caution in patients with diagnosed or suspected cardiac problems or epilepsy.
Full prescribing information can be found in product labeling or at: www.bionessrehab.com/l360/safety-information.
Instructions for Use / Patient Guides and Reference Cards can be made available upon request.
Contact [email protected] or 855-902-5252 to request an electronic copy.