How Does It Work?
How Does the L300 Go Work?
The L300 Go’s accelerometer and three-axis gyroscope monitor the user’s movement. Using the information it gathers, the L300 Go detects gait irregularities and then deploys corrective nerve stimulation in 0.01 seconds. This helps users lift their feet fully and correctly on various surfaces—including stairs, ramps, and uneven terrain—at different walking speeds.
Quick, Intuitive Setup
The L300 Go has only a few components to manage, plus a Quick Start Fitting Mode and Bluetooth® programming. The design minimizes setup time, so users can be on their feet and walking sooner.
- Minimal components: 3D motion detection and onboard controls make the foot sensor and control unit optional.
- Onboard controls: Onboard controls ease utilization, with the abilities to power on and off, change the functional mode, and manage intensity levels based on clinician specifications.

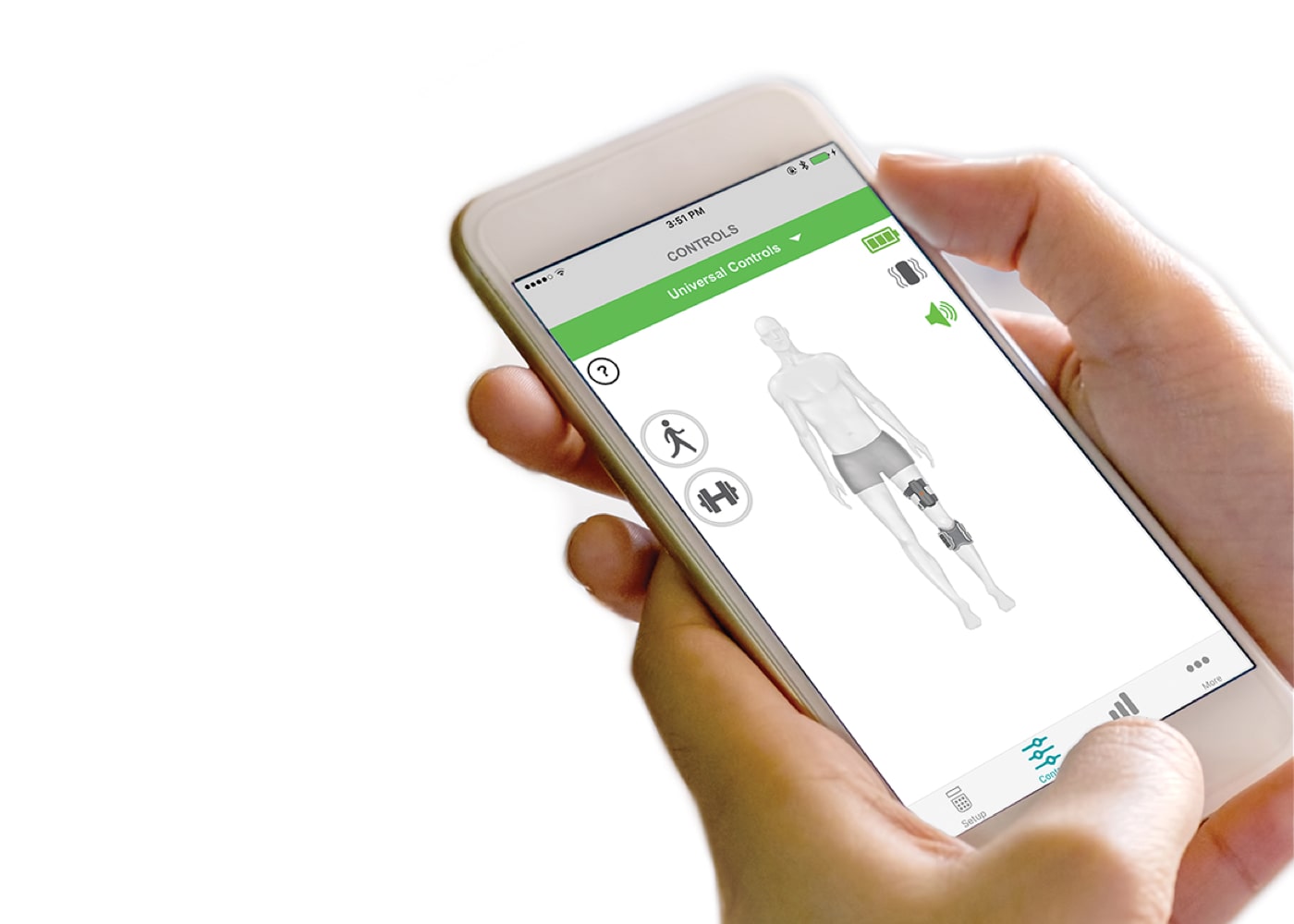
User App
The myBioness™ mobile app continues therapeutic gains by letting users control their L300 Go, set goals, and monitor their activity and progress.
More Questions?
Have questions about L300 Go? Check out common questions and concerns on the FAQs page.
Contact us to schedule a demo with L300 Go or if you have questions about other Bioness Medical, Inc. products.
Indications for Use: The L300 Go System is intended to provide ankle dorsiflexion in adult and pediatric individuals with foot drop and/or to assist knee flexion or extension in adult individuals with muscle weakness related to upper motor neuron disease/injury (e.g., stroke, damage to pathways to the spinal cord). The L300 Go System electrically stimulates muscles in the affected leg to provide ankle dorsiflexion of the foot and/or knee flexion or extension; thus, it also may improve the individual’s gait.
The L300 Go System may also facilitate muscle re-education, prevent/retard disuse atrophy, maintain or increase joint range of motion, and increase local blood flow.
L300 Go is contraindicated in patients with a demand-type cardiac pacemaker, defibrillator or any electrical implant. Do not use the system on a leg where metallic implant is directly underneath the electrodes, a cancerous lesion is present or suspected, or on a leg with regional disorder (e.g., fracture or dislocation) which could be adversely affected by motion from the stimulation. Use caution in patients with diagnosed or suspected cardiac problems or epilepsy.
Full prescribing information can be found in product labeling or at: www.bionessrehab.com/l300/safety-information.
Instructions for Use / Patient Guides and Reference Cards can be made available upon request.
Contact [email protected] or 800-211-9136 to request an electronic copy.

