Success Stories

After a Brain Injury, Mike
Uses the L300 Go to Regain Mobility
His Story
As the father of four children, and an avid cyclist and rock climber, Mike was always able to stay active and in great shape. Then during a bike ride one evening, he was struck by a car and suffered a traumatic brain injury (TBI).
When Mike awoke from the coma, he began intensive rehabilitation to relearn everyday tasks such as walking. Even with his immense determination, though, Mike knew he had a daunting recovery ahead.
His Solution
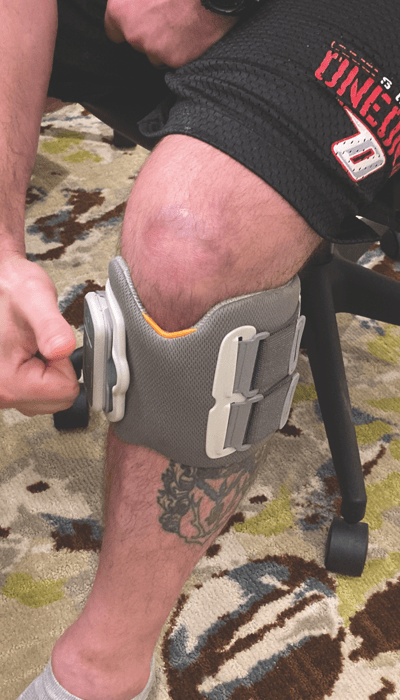
Finding the Missing Piece
Initially, Mike used a rigid ankle-foot orthosis (AFO) to help correct his foot drop. While the AFO helped him walk, it didn’t improve the leg muscle strength he needed to move forward in his recovery.
It was during his first talk with a new physical therapist that Mike learned about the L300 Go Foot Drop System. Happy to try any alternative to the AFO, Mike put on the L300 Go and soon knew it was the missing piece of his rehabilitation puzzle.
His Next Steps
Taking Major Steps Forward
Mike was surprised by how smoothly he moved with the L300 Go. Crucially, it also allowed him to move his ankle, begin rebuilding strength in his leg, and pivot effortlessly, all major steps in his recovery.
Mike knows life may never be the same as before his TBI, but the horizon is brighter these days. He can get on the floor and play with his children, an important goal for him. And on those occasions when Mike needs a boost, his wife Dani reminds him of the tremendous progress he has made–thanks to his family, his rehabilitation team, lots of hard work, and the L300 Go.

See more Success Stories
See how the L300 Go has changed other patients’ lives. Check out their success stories.
Contact us to schedule a demo with L300 Go or if you have questions about other Bioness Medical, Inc. products.
Indication for Use: The L300 Go System is intended to provide ankle dorsiflexion in adult and pediatric individuals with foot drop and/or to assist knee flexion or extension in adult individuals with muscle weakness related to upper motor neuron disease/injury (e.g., stroke, damage to pathways to the spinal cord). The L300 Go System electrically stimulates muscles in the affected leg to provide ankle dorsiflexion of the foot and/or knee flexion or extension; thus, it also may improve the individual’s gait.
The L300 Go System may also facilitate muscle re-education, prevent/retard disuse atrophy, maintain or increase joint range of motion, and increase local blood flow.
L300 Go is is contraindicated in patients with a demand-type cardiac pacemaker, defibrillator or any electrical implant. Do not use the system on a leg where metallic implant is directly underneath the electrodes, a cancerous lesion is present or suspected, or on a leg with regional disorder (e.g., fracture or dislocation) which could be adversely affected by motion from the stimulation. Use caution in patients with diagnosed or suspected cardiac problems or epilepsy. Full prescribing information can be found in product labeling or at: www.bionessrehab.com/l300/safety-information.

