Success Stories

Managing Multiple Sclerosis While Maintaining Her Career,
Deb Powers Through It All
Her Story
Deb was just 34 when she was diagnosed with multiple sclerosis (MS). By then, she was years into a distinguished career in criminal justice. Deb was also physically active, spending her recreational time hiking and horseback riding.
At first, Deb was able to compensate for her symptoms. But in 2017, at age 57, she was diagnosed with progressive secondary MS, which is marked by a worsening of neurologic function.
Her Solution
Device Makes a Difference
Mobility challenges mostly had affected Deb’s left side, but the MS increasingly affected her right side, too. For the first time, she experienced foot drop in her right leg, as well as difficulty with gross and fine motor movements in her right arm.
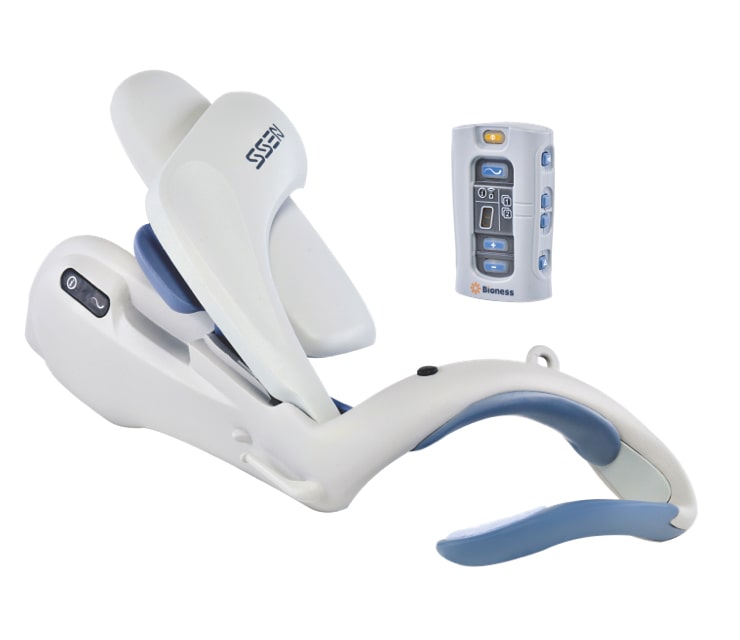
Deb first learned about the L300 Go Foot Drop System and the H200 Wireless Hand Rehabilitation System during physical therapy. Using both devices, she started to feel more control and independence.
Her Next Steps
New Hope and Encouragement
Deb was so encouraged that she obtained her own L300 Go and the H200 Wireless devices to use outside of the therapy clinic. For the first time in too long, Deb could perform daily tasks and get around on her own.
“I come from a long line of healthy people who stayed healthy until they were very old,” Deb said. “If I didn’t have MS, I’d be doing everything I did before, even in my 60s. I’d like to experience recovery, and using the Bioness devices has given me hope that I could get there.”
See more Success Stories
See how the H200 Wireless Hand Rehabilitation System has changed other patients’ lives. Check out their success stories.
Contact us to schedule a demo with H200 Wireless or if you have questions about other Bioness products.
Indication for Use
The H200 Wireless System is an electrical stimulation device indicated for the following uses:
- Functional Electrical Stimulation (FES)
Improvement of hand function and active range of motion in patients with hemiplegia due to stroke or upper limb paralysis due to C5 spinal cord injury. - NeuroMuscular Electrical Stimulation (NMES)
Maintenance and/or increase of hand range of motion; prevention and/or retardation of disuse atrophy; increase in local blood circulation; reduction of muscle spasm; re-education of muscles.
Full prescribing information can be found in product labeling or at: www.bionessrehab.com/h200/safety-information/.

