L300 Go Resources
Select any L300 Go resource below to open it.
L300 Go: User’s Guide
- Safety information
- Setup, operation, and maintenance
- Full details about the L300 Go
L300 Go Lower Cuff: User’s Reference Card
- Putting on the cuff
- Charging the batteries
- Message displays
L300 Go Thigh Cuff Stand-Alone: User’s Reference Card
- Putting on the cuff
- Charging the batteries
- Message displays
L300 Go: Warranty
- Terms and conditions
- Consumer/purchaser responsibilities
- Exclusions, disclaimers, and limitation of liability
myBionessTM App: Phone Compatibility List
- Android operating system versions
- Apple iPhone versions
- Compatibility FAQs
L300 Go Clinician’s Guide
Product Brochure
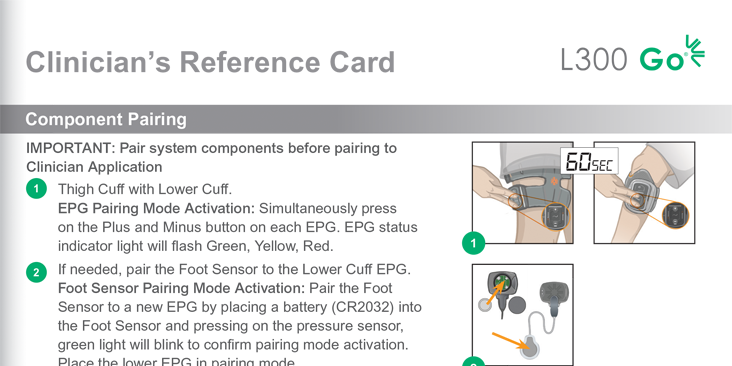
L300 Clinician’s Reference Card
Patient Consent Form
L300 Go Patient First Impressions
L300 Go Technology Introduction
L300 Go Information Sheet
myBioness™ User Guide
Instructions for pairing and using the mobile app
Technical Support
Nothing found.
Find a Facility Near You
Check out our facility finder to locate the closest facilities that use Bioness technology in their rehabilitation programs.
Contact us to schedule a demo with L300 Go or if you have questions about other Bioness products.
Indication for Use: The L300 Go System is intended to provide ankle dorsiflexion in adult and pediatric individuals with foot drop and/or to assist knee flexion or extension in adult individuals with muscle weakness related to upper motor neuron disease/injury (e.g., stroke, damage to pathways to the spinal cord). The L300 Go System electrically stimulates muscles in the affected leg to provide ankle dorsiflexion of the foot and/or knee flexion or extension; thus, it also may improve the individual’s gait.
The L300 Go System may also facilitate muscle re-education, prevent/retard disuse atrophy, maintain or increase joint range of motion, and increase local blood flow.
L300 Go is is contraindicated in patients with a demand-type cardiac pacemaker, defibrillator or any electrical implant. Do not use the system on a leg where metallic implant is directly underneath the electrodes, a cancerous lesion is present or suspected, or on a leg with regional disorder (e.g., fracture or dislocation) which could be adversely affected by motion from the stimulation. Use caution in patients with diagnosed or suspected cardiac problems or epilepsy.
Full prescribing information can be found in product labeling or at: www.bionessrehab.com/l300/safety-information.
Instructions for Use / Patient Guides and Reference Cards can be made available upon request.
Contact [email protected] or 800-211-9136 to request an electronic copy.