Success Stories
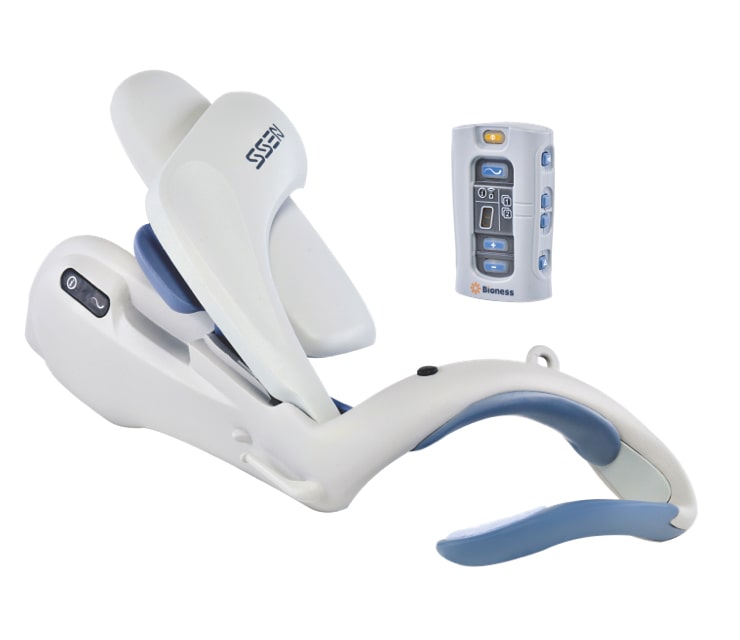
See how the H200 Wireless Hand Rehabilitation System has changed patients’ lives.
Watch Patient Testimonials
Read Patient Testimonials
See Additional Resources
Check out our wide variety of resources to learn more about the H200 Wireless Hand Rehabilitation System.
Contact us to schedule a demo with H200 Wireless or if you have questions about other Bioness products.
Indications for Use:
The H200 Wireless System is an electrical stimulation device indicated for the following uses:
- Functional Electrical Stimulation (FES)
- Improvement of hand function and active range of motion in patients with hemiplegia due to stroke or upper limb paralysis due to C5 spinal cord injury.
- NeuroMuscular Electrical Stimulation (NMES)
- Maintenance and/or increase of hand range of motion; Prevention and/or retardation of disuse atrophy; Increase in local blood circulation; Reduction of muscle spasm; Re-education of muscles.
H200 Wireless is contraindicated in patients with a demand-type cardiac pacemaker, defibrillator or any electrical implant. Do not use the system on a hand where metallic implant is directly underneath the electrodes, a cancerous lesion is present or suspected, or on a hand with regional disorder (e.g., fracture or dislocation) which could be adversely affected by motion from the stimulation. Use caution in patients with diagnosed or suspected cardiac problems or epilepsy.
Full prescribing information can be found in product labeling or at bionessrehab.com/ous/h200/safety-information/.
Instructions for Use / Patient Guides and Reference Cards can be made available upon request.
Contact [email protected] to request an electronic copy.